Tackling Trauma: A Revolutionary Approach to Emergency Medical Care – Dr Kayvan Najarian, University of Michigan
Jun 29, 2018health and medicine
The multiple injuries sustained in a traumatic accident put victims’ lives at risk and can be difficult to diagnose accurately in fast paced emergency room settings. Dr Kayvan Najarian and his team of researchers at the University of Michigan are designing advanced automated trauma decision support systems that can help emergency physicians make critical time-sensitive decisions in life or death situations.

You may also like …

Prof. Diana Jaalouk | Editing DNA and Degrading Proteins: The Tools to Achieve Precision Oncology
Cancer is a daunting healthcare challenge, and is still affecting millions worldwide, despite the enormous research resources that have been directed at finding effective treatments over the past decades. Many anti-cancer treatments remain poorly specific for the tumours they are intended to treat, and often suffer from modest efficacy and serious off-target effects. Part of the problem is the inherent variability between many tumours and their resulting unpredictable responses to standard chemotherapy. However, the latest advancements in precision oncology may be the start of a new paradigm, potentially providing targeted therapeutic payloads that can successfully address the specific and unique issues underlying a given patient’s cancer. Researchers such as Prof. Diana Jaalouk and her colleagues at the American University of Beirut in Lebanon are pioneering innovative tools that are changing the way we understand and treat this complex disease. Two remarkable recent technologies, CRISPR-Cas9 and PROteolysis TArgeting Chimeras (or PROTACs for short), are at the forefront of this precision revolution. While distinct in their approach, these tools share a common goal: targeting cancer with precision and minimizing harm to healthy cells. Together, they are set to reshape the therapeutic landscape.

Dr. Adeniyi Charles Adeola | Beyond Chickens: Unlocking the Hidden Treasures of Nigeria’s Poultry
When most of us think about poultry, our minds often turn to chickens, the staple of farms and dinner tables worldwide. However, Nigeria is home to several other fascinating types of poultry beyond the humble chicken that have played significant roles in the country’s agriculture, culture, and economy. While these poultry species are firmly embedded in the Nigerian agricultural system, the history of how and when these animals came to be domesticated and where these populations originally derived from is often obscure. Recent research conducted by Dr. Adeniyi Charles Adeola of the Chinese Academy of Sciences, and colleagues, has shed new light on three often-overlooked poultry species, the Muscovy duck, the domestic pigeon, and the helmeted guinea fowl. These birds not only offer valuable genetic resources but also hold keys to food security, sustainable farming, and biodiversity conservation.

Dr. Erin Berthold | Plant-Based but Powerful: The Hidden Interactions Between Kratom and CBD
In recent years, natural products such as kratom, which derives from a Southeast Asian tree called Mitragyna speciosa, and cannabidiol (or CBD) which derives from the Cannabis plant, have gained significant popularity for their potential to relieve anxiety, manage pain, and enhance mood. While both substances are often praised by users for their plant-based origins, and are often considered safer than synthetic pharmaceuticals as a result, the scientific community is working to uncover the complexities behind how these compounds interact, not just with the human body but with each other. After all, plant-based compounds are still active, and have the same potential for benefit and harm as any drug. People who use kratom are also more likely to use CBD, meaning that they could potentially experience a drug interaction if both substances are ingested around the same time. A recent study by Dr. Erin Berthold and her colleagues at the University of Florida sheds new light on the pharmacokinetic interactions between kratom and CBD, revealing findings that are both fascinating and important for public health.
Dr. Ben Sorum | The Brain’s Hidden Switches: The Power of Ultrasound in Neural Modulation
We think of our brains as safe and secure within our skulls, and not easily influenced unless we consume a mind-altering substance, suffer a traumatic injury or undergo invasive brain surgery. However, recent research shows that our brain activity can be influenced non-invasively using nothing but sound and that this technique could have therapeutic potential. As a postdoctoral researcher at UC Berkeley, Dr. Ben Sorum began to think about these types of question while in the Lab of Dr. Stephen G. Brohawn. Now, Dr. Sorum’s current research at Cooper Medical School of Rowan University explores how ultrasound, which can be non-invasively administered from outside the brain and through the skull, can activate specialized proteins in brain cells, changing their activity. The technique, if further developed, may play a key role in the future of neuromodulation, a field with enormous potential for treating neurological disorders.

Dr. Christopher Marinangeli | The Power of Plants: Making the Most of Plant-Based Proteins
In recent years, plant-based diets have gained significant traction, not just among vegetarians and vegans but also among individuals looking to improve their health and reduce their environmental impact. Increasing public awareness of the role of animal food production in driving climate change, along with the potential health risks of consuming large amounts of animal foods has powered this phenomenon. However, one of the ongoing debates in nutrition revolves around protein, a crucial nutritional component, and the nutritional quality of various protein sources. Can plant-based protein sources provide sufficient, high-quality protein compared with animal-based protein sources in the context of a dietary pattern? The question relates to consumer awareness and education, as not all plant proteins are created equal, and replacing meat, diary, and other animal proteins with just one or two plant protein sources may not provide everything we need nutritionally. Rather, a mix of plant protein sources may be required as an adequate replacement for high quality animal protein. As consumers increasingly replace animal proteins with plant proteins, potentially without awareness of these issues, is the overall quality of the protein they are consuming decreasing? Dr. Christopher Marinangeli of Protein Industries Canada and his colleagues set out to answer this question in their research on the effects of increasing plant protein intake on protein quality and nutrient consumption among U.S. adults.

Dr. Adeniyi Charles Adeola | The Genetic Blueprint of Nigerian Animals: How Genetics Research is Transforming Nigerian Wildlife and Farming
Across the varied and diverse landscapes that make up the Nigerian countryside, animals, both wild and domesticated, are more than merely an agricultural commodity or source of food; they are an integral part of local cultures, natural biodiversity, and represent an ecological treasure trove. Local wildlife and agricultural livestock help to sustain the livelihoods of millions. However, beyond this, Nigerian animals hold secrets within their genetic code that could, when revealed, help to prevent diseases, aid conservation efforts and enhance agricultural productivity. Leading the efforts to uncover useful and interesting genetic phenomena in these animals is Dr. Adeniyi Charles Adeola of the Chinese Academy of Sciences, who explores the genetic blueprints of Nigerian animals in his pioneering research. From investigating the population dynamics of grasscutters to tackling the genetic roots of prion diseases, Dr. Adeniyi Charles Adeola’s work illuminates both challenges and solutions that impact food security, agriculture, and biodiversity in Nigeria, and far beyond.

Professor Magnus S. Magnusson | The surprising similarities between the structures of human cells and societies
Research by Professor Magnus S. Magnusson at the University of Iceland demonstrates surprising similarities between the organization of cellular protein networks and of human societies. He reveals how the invention of writing and, very recently, general education, transformed human civilization in ways that mirror ancient biological developments and emphasises how this makes humans unique.
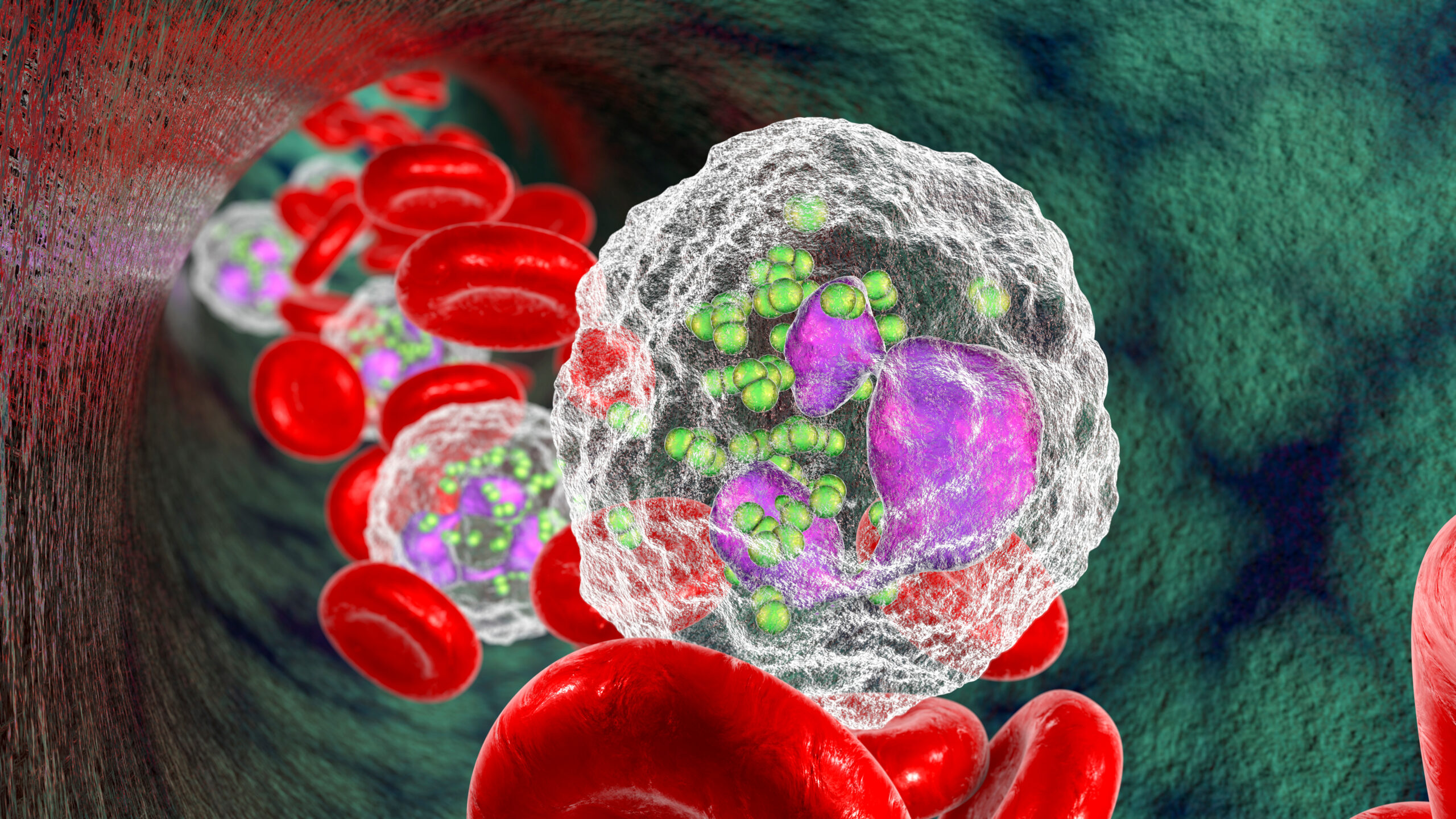
Dr. Roberta Martinelli | Sepsis and the Silent Battle Within: Neutrophils’ Role in Sepsis-Related Complications
Sepsis is a critical illness that begins with a simple infection and degenerates into a severe and dysregulated immune response that affects the whole body. This significant immune reaction typically causes widespread inflammation and can progress very rapidly. This can result in serious damage to tissues and organs, potentially leading to organ failure and death. Despite the severity of sepsis and its frequent poor prognosis, effective treatments are still elusive, and many sepsis patients remain at high risk of death and serious complications. Part of the issue is the complex cascade of cellular and biochemical events that underlie sepsis, which has made it difficult to obtain a comprehensive overview of the illness from which to design an effective treatment. Dr. Roberta Martinelli, Executive Director of Stromal Immunology and Early Discovery, Discovery Immunology, Merck, and colleagues, have published a study in the journal iScience which reveals new insights into the complex biological milieu underlying sepsis, and uncovers pathways and potential treatment targets that could change how we diagnose and treat this life-threatening illness.
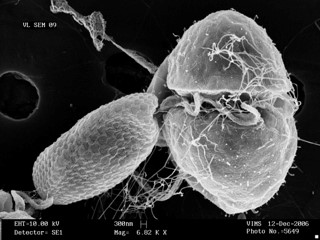
Dr. Allen Place | Small but Deadly: The Tale of K. veneficum
The oceans, huge and brimming with diverse lifeforms, pose no less a struggle for survival for their inhabitants than that faced by creatures on dry land. Evolution has furnished marine organisms with huge array of defensive, and indeed, offensive adaptations to help them to thrive in this battleground. Among the organisms who live and compete in the ocean are dinoflagellates. These are small, single-celled creatures that are an important component of plankton found in marine ecosystems. Despite their tiny size, dinoflagellates such as Karlodinium veneficum can wield potent biochemical weaponry that gives them an edge against other competing organisms. Decades since the discovery of the toxic properties of Karlodinium veneficum, researchers such as Dr. Allen Place of the University of Maryland Center for Environmental Sciences, and his colleagues, have begun to unravel the secrets of its potent toxins, called karlotoxins. Their findings offer fascinating insights into the interactions of marine life and the weapons they adopt to capture prey and deter predators.

Dr. Andrea Grindeland | The Tiny Heroes That Could Save Deer and Elk from Chronic Wasting Disease
It’s not difficult to picture a lush forest landscape populated with majestic deer and elk, long admired for their prowess and strength. Now, imagine that same scene, but instead of healthy and happy animals browsing a forest ecosystem, we see creatures that are thin and disoriented, that struggle to run or even stand, with halting and confused movements that are pitiable and distressing to watch. This is the harsh reality of Chronic Wasting Disease, an illness that currently has no cure and that threatens such wildlife around the world. Part of the challenge with Chronic Wasting Disease is the difficulty in studying it reliably in wildlife. The disease has subtle signs at an early stage, and it is difficult to obtain robust and reproducible data from large, wild animals who often live in remote and poorly accessible forest ecosystems. Consequently, researchers have turned to an unlikely but powerful ally, the tiny laboratory mouse, to model and study the disease under laboratory conditions. Dr. Andrea Grindeland of the McLaughlin Research Institute, and her colleagues, have authored a review of the existing mouse models of Chronic Wasting Disease. These tiny creatures have been engineered to mimic the biology of cervids, such as deer and elk, and are providing crucial insights into how Chronic Wasting Disease evolves, is transmitted, and how it might one day be controlled or even eradicated.