Health Neuroscience How and Why Exercise Improves Cognitive – Professor Kirk Erickson, University of Pittsburgh
Original Article Reference
https://doi.org/10.33548/SCIENTIA478
Share Episode
About this episode
We all know exercise is good for us. In addition to the renowned physical benefits, Professor Kirk Erickson in the Department of Psychology at the University of Pittsburgh is providing powerful evidence that exercise may improve cognitive faculties throughout the lifespan. Read on to discover the wide range of ways in which exercise can help us to live our lives to the fullest across the years, and how the emerging field of health neuroscience may inform public health policy for our better good.
This work is licensed under a Creative Commons Attribution 4.0 International License. 
What does this mean?
Share: You can copy and redistribute the material in any medium or format
Adapt: You can change, and build upon the material for any purpose, even commercially.
Credit: You must give appropriate credit, provide a link to the license, and indicate if changes were made.
Related episodes
Dr. Andrea Grindeland | The Tiny Heros That Could Save Deer and Elk from Chronic Wasting Disease
It’s not difficult to picture a lush forest landscape populated with majestic deer and elk, long admired for their prowess and strength. Now, imagine that same scene, but instead of healthy and happy animals browsing a forest ecosystem, we see creatures that are thin and disoriented, that struggle to run or even stand, with halting and confused movements that are pitiable and distressing to watch. This is the harsh reality of Chronic Wasting Disease, an illness that currently has no cure and that threatens such wildlife around the world. Part of the challenge with Chronic Wasting Disease is the difficulty in studying it reliably in wildlife. The disease has subtle signs at an early stage, and it is difficult to obtain robust and reproducible data from large, wild animals who often live in remote and poorly accessible forest ecosystems. Consequently, researchers have turned to an unlikely but powerful ally, the tiny laboratory mouse, to model and study the disease under laboratory conditions. Dr. Andrea Grindeland of the McLaughlin Research Institute, and her colleagues, have authored a review of the existing mouse models of Chronic Wasting Disease. These tiny creatures have been engineered to mimic the biology of cervids, such as deer and elk, and are providing crucial insights into how Chronic Wasting Disease evolves, is transmitted, and how it might one day be controlled or even eradicated.
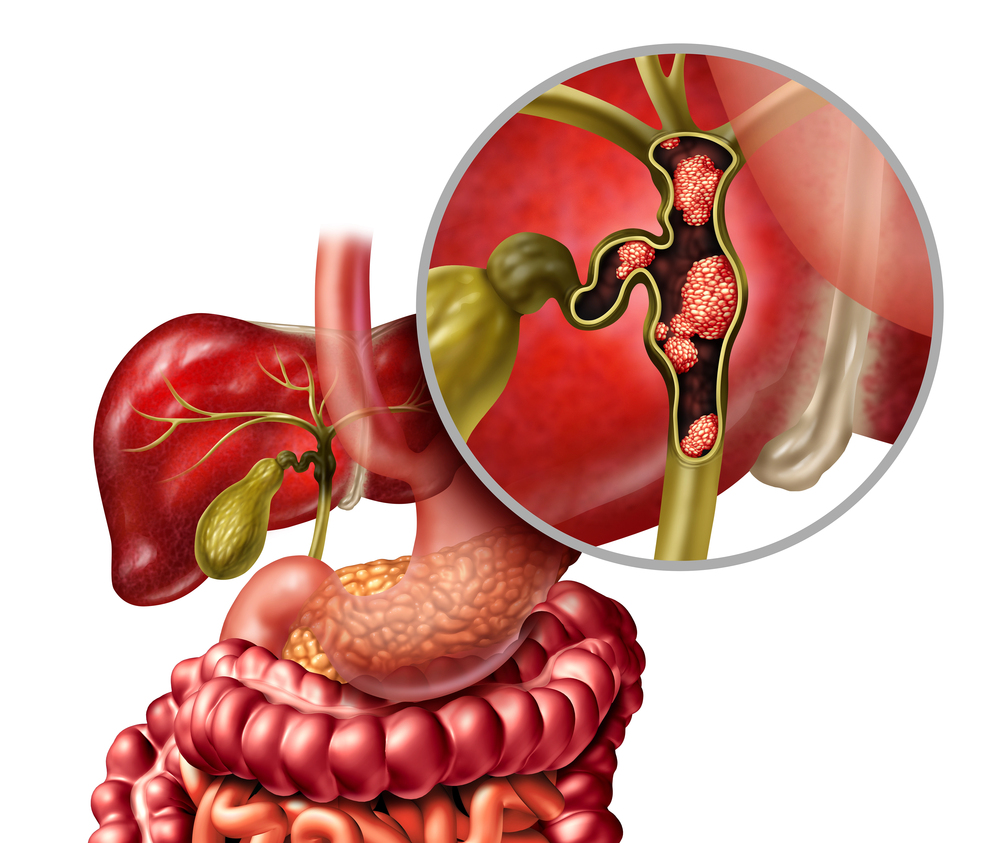
Dr. Kim Saverno | Real-World Impact: How Targeted Therapy is Changing Cholangiocarcinoma Treatment
Cholangiocarcinoma is an aggressive cancer that begins in the bile ducts. While the cancer is relatively rare, affecting approximately 8,000 people in the United States each year, unfortunately, it is often undiagnosed until an advanced stage. This late diagnosis makes cholangiocarcinoma very challenging to treat, and less than 10% of patients survive for five years after diagnosis. Traditional anti-cancer treatments, such as chemotherapy, have only limited effectiveness in cholangiocarcinoma, and can cause serious side effects. Recently developed immunotherapy and targeted therapies have provided promising options for this difficult-to-treat disease. Dr. Kim Saverno of the Incyte Corporation, a biopharmaceutical company headquartered in the U.S., and the study’s co-authors, have been studying the real-world use of an FDA-approved targeted drug for cholangiocarcinoma called pemigatinib. Pemigatinib was approved by the FDA in 2020. It can be specifically used for cholangiocarcinoma patients who have genetic changes in a protein known as fibroblast growth factor receptor 2, or FGFR2 for short. Their study, to the best of our knowledge, is the first to examine pemigatinib’s use in real-world settings, and reveals information about treatment patterns, FGFR2 testing patterns, and effectiveness of pemigatinib for cholangiocarcinoma when used in everyday practice.
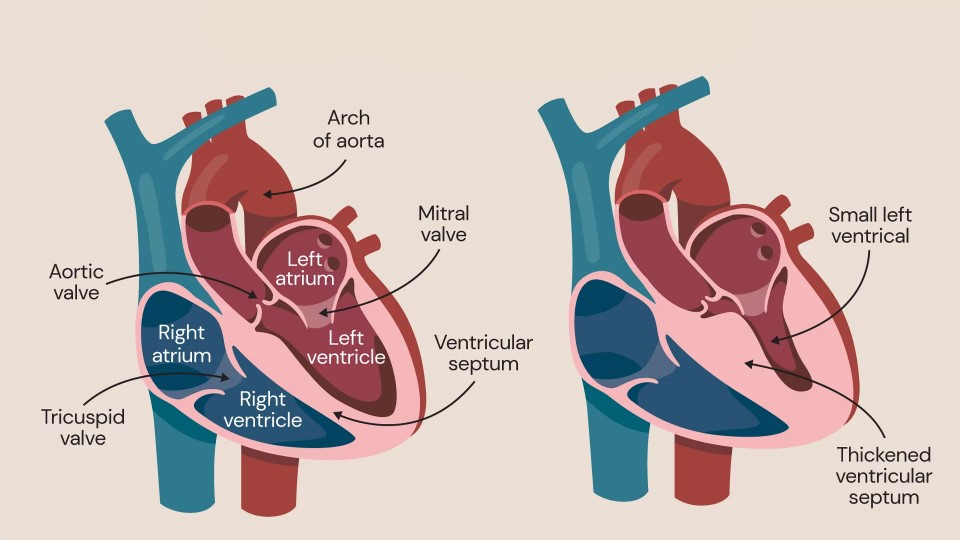
Dr Richard Saumarez | Beyond the Heartbeat: How Direct Cardiac Investigation Could Save Lives in Hypertrophic Cardiomyopathy and Beyond
Hypertrophic cardiomyopathy (or HCM for short) is a serious heart condition that involves thickening of the heart muscle wall and disruption of the normal tissue architecture, called ‘disarray’. This can result in sudden cardiac death caused by abnormal heart rhythms, known as arrhythmias. Identifying those HCM patients who are most at risk could permit preventative measures, such as implanting a cardioverter-defibrillator, which could potentially be lifesaving. However, current techniques to predict the risk of sudden death in HCM are limited, leaving patients underserved. In a recent study, Dr Richard Saumarez, an academic cardiologist formerly of the University of Cambridge, and colleagues, questioned whether conventional methods, which consider risk factors such as family history of sudden death or the degree of heart muscle thickening, are effective in predicting sudden death in HCM patients. Their research suggests that risk factor assessments might miss crucial information about the heart’s electrical behaviour, which could provide more accurate clues about the risk of sudden death. As an alternative, the researchers propose direct heart-investigation methods, called electrophysiological techniques, as a more reliable assessment. Although the study was concerned with HCM, the arguments put forward are more general and applicable to other diseases, particularly to survivors of myocardial infarcts who are also at risk of sudden death.
Dr. Qiang Wang | Fishing for Findings: Uncovering the Genetics of Hearing Loss
Our hearing is amongst our most profound senses, connecting us to the surrounding world through sound. However, this connection is diminished or absent altogether in millions of people around the world because of hearing loss. Hearing loss is a common sensory disorder and is often hereditary. The condition can be caused by complex genetic factors, and so far, researchers have linked over 150 genes to hearing impairment. Now, a new collaborative study led by Dr. Qiang Wang of the South China University of Technology, Dr. Tao Cai from the National Institute of Health, and Dr. Yuan Li from the China-Japan Friendship Hospital in Beijing, has uncovered a new genetic clue, a mutation in the OXR1 gene, that could upend our understanding of hereditary hearing loss, and the eventual treatments that we develop to combat it.
Increase the impact of your research
• Good science communication encourages everyday people to be scientifically literate so that they can analyse the integrity and legitimacy of information.
• Good science communication encourages people into STEM-related fields of study and employment.
• Good public science communication fosters a community around research that includes both members of the public, policymakers and scientists.
• In a recent survey, 75% of people suggested they would prefer to listen to an interesting story than read it.
Step 1 Upload your science paper
Step 2 SciPod script written
Step 3 Voice audio recorded
Step 4 SciPod published